#52,000
#68,300
#68,800
#69,300
Correct answer is C
opening stock 800
purchase 100,000
100,800
less; closing stock 1500 99,300
Salaries 30,000
Rates(1:2 of 1500= 1/3x1500) 500 30500
68,800
If humid air is polluted by chlorine discharged, the air can be restored by sprinkling
solid MnO\(_{2}\)
acidified KMnO\(_{4}\)
acidified FeSO\(_{4}\)
saturated NaCl(aq)
Correct answer is B
Chlorine is a toxic gas and when it pollutes the air, it can be neutralized by using acidified KMnO4. The reaction between chlorine and acidified KMnO4 results in the formation of chloride ions, manganese(II) ions, and water, which are harmless. This is why acidified KMnO4 is used to restore air polluted by chlorine discharge.
Cathodic protection of metals is based on
standard electrode potential of hydrogen
its electrical conductivity
nature of oxides formed
relative tendencies of oxidation
Correct answer is D
No explanation has been provided for this answer.
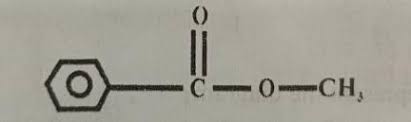
What is the relative molecular mass of the compound below? [H = 1.0; C = 12.0; O = 16.0]
137
136
64
59
Correct answer is B
The molecular formula of the compound in question is \(C_6H_5COOCH_3\)
Then its relative molecular mass = 8C + 8H + 2O = 8 X 12 + 8 x 1 + 2 X 16 = 136
C\(_{3}\)H\(_{6}\)
C\(_{4}\)H\(_{8}\)
C\(_{2}\)H\(_{4}\)
CH\(_{2}\)
Correct answer is B
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests