electrolyte
electrodes
salt bridge
solid - liquid interface
Correct answer is C
No explanation has been provided for this answer.
Rate of backward reaction will increase
The equilibrium constant will shift
Rates of forward and backward reactions are not affected
Rate of forward reaction will increase
Correct answer is A
The given reaction is endothermic, hence, a decrease in temperature will favor the exothermic side which is the backward reaction.
The following are isoelectronic ions except
Na\(^+\)
Mg\(^{2+}\)
Si\(^{2+}\)
O\(^{2+}\)
Correct answer is C
Two or more ions are said to be isoelectronic if they have the same electronic structure and the same number of valence electrons.
Na\(^+\) = 10 electrons = 2, 8
Mg\(^{2+}\) = 10 electrons = 2,8
O\(^{2-}\) = 10 electrons = 2,8
Si\(^{2+}\) = 12 electrons = 2,8,2
\(\implies\) Si\(^{2+}\) is not isoelectronic with the rest.
The hybridization in the compound \(CH_3 - CH_2 - C \equiv H\) is
sp\(^3\) and sp
sp
sp\(^2\)
sp\(^3\) and sp\(^2\)
Correct answer is A
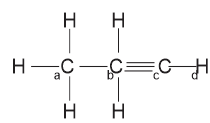
The hybridization in a and b is sp\(^3\) hybridization while in c and d is sp hybridization.
Z\(_3\)(SO\(_4\))\(_2\)
ZSO\(_4\)
Z\(_2\) SO\(_4\)
Z\(_2\)(SO\(_4\))\(_3\)
Correct answer is D
Z = 1s\(^2\) 2s\(^2\) 2p\(^6\) 3s\(^2\) 3p\(^1\)
\(\therefore\) We have Z\(^{3+}\) and SO\(_4 ^{2-}\)
The reaction : Z\(^{3+}\) + SO\(_4 ^{2-}\) \(\to\) Z\(_2\)(SO\(_4\))\(_3\).
JAMB Subjects
Aptitude Tests