7.45 g
14.90 g
74.50 g
149.00 g
Correct answer is D
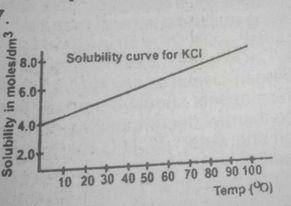
Trace the graph to the y-axis to obtain the solubility of the salt at 80°C and at the point where the straight line touches the y-axis, the solubility of the salt at these respective temperatures is 4mol\dm3 and 6mol\dm3 solubility of salt that will crystallize out = 6- 4 = 2mol\dm3 therefore mass of salt deposited = solubility x molar mass of salt 2 x 74.5 = 149.00g
300, 500
500, 300
-300, -500
-500, -300
Correct answer is C
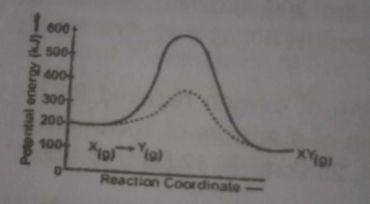
The uncatalysed reverse reaction = 100 - 400 = -300 The calalysed reverse reaction = 100 - 600 = - 500
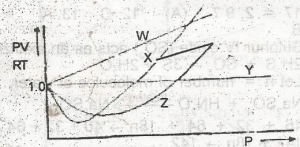
Which of the curves in the above graph illustrates the behaviour of an ideal gas?
W
X
Y
Z
Correct answer is C
No explanation has been provided for this answer.
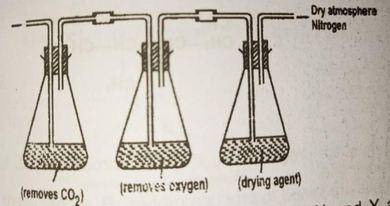
In the above set up substances X and Y are respectively
lime water copper (ll) tetraoxosulphate (lV)
potassium trioxocarbonate and alkaline pyrogallol
potassium hydroxide and alkaline pyrogallol
potassium trioxocarbonate (lV) and concentrated tetraoxosulphate (IV) acid
Correct answer is C
No explanation has been provided for this answer.

It can be deduced from the vapour pressure curves above that
liqiud 1 has the highest boiling point
liquid ll has the highest boiling point
liquid lll has the highest boiling point
liquid lll has the lowest boiling point
Correct answer is C
Trace the iii curve horizontally and you have over 20°C temperature
JAMB Subjects
Aptitude Tests