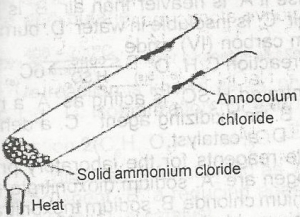
evaporation
recrystallization
sublimation
fractional precipitation
Correct answer is C
No explanation has been provided for this answer.
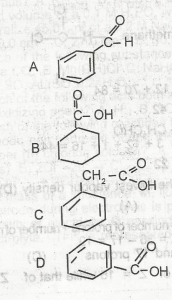
Use the option above to answer this question. The structure of benzoic acid is
A
B
C
D
Correct answer is D
No explanation has been provided for this answer.

for a 10o rise in temperature, rate of reaction is double
for a 10 o rise in temperature, rate of reaction is halved
time taken for iodine to appear does not depend on temperature
for a 10 o rise in temperature rate of reaction is tripled
Correct answer is B
The iodine liberation process is significantly affected by the amount of acid, that of potassium iodide added, the waiting time for the liberation, and light; therefore, the process plays a key role for the accuracy of the titration results.
for a 10 o rise in temperature, rate of reaction is halved

A
B
C
D
Correct answer is A
lime juice reacts with NaCO3 with the evolution of CO2 because it is an acid while NaCO3 with ethanol does not result in a significant chemical reaction.
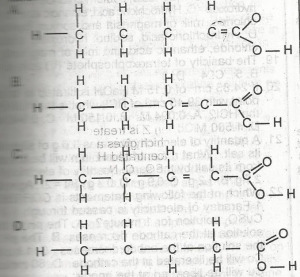
A
B
C
D
Correct answer is C
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests