Hydrogen can be displaced from a hot alkaline solution by
Fe
Cu
Ca
Sn
Correct answer is D
Tin dissolves in concentrated solution of alkalis to give trioxostannate(IV) salt and hydrogen.
15.3cm3
87.3cm3
2.0cm3
97.0cm3
Correct answer is D
\(\frac{V_{1}}{T_{1}}\) = \(\frac{V_{2}}{T_{2}}\)
\(T_{1}\) = 3 + 273 = 276K
\(T_{2}\) = 18 + 273 = 291K
\(V_{1}\) = \(92cm^{3}\)
\(V_{2}\) = ?
\(V_{2}\) = \(\frac{V_{1}T_{2}}{T_{1}}\)
=\(\frac{92 \times 291}{276}\)
= \(97.0cm^{3}\)
A liquid that will dissolve fat is
hydrochloric acid
calcium hydrochloride
kerosene
water
Correct answer is C
Kerosene is only liquid that will dissolve fat. Option A,B,D will not dissolve fat because of their chemical composition
Which of the following metals burns with brick red
Pb
Ca
Na
Mg
Correct answer is B
Calcium is the only metal that burn with brick red flame. Option A,C,D will not burn with brick red flame rather burn with brick blue flame
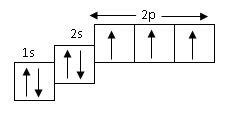
The above orbital diagram shown the electronic configuration of
chlorine
nitrogen
calcium
neon
Correct answer is B
Nitrogen has an electronic configuration of 7 i.e
1s2 2ps2 2p3. option A,C,D are chlorine 17, calcium 20, neon 10
JAMB Subjects
Aptitude Tests