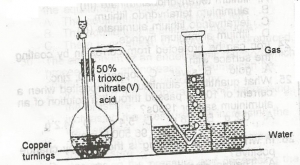
In the diagram above, the gas produced is?
NO
NO2
N2O
N2O4
Correct answer is B
In this laboratory preparation, 50% trioxonitrate (V) acid is reacted with copper turnings to liberate nitrogen (II) oxide ( NO ) that is collected by downward delivery.
Cu+\(4HNO_3 →Cu(NO_3)_2+2NO_2+2H_2O\)
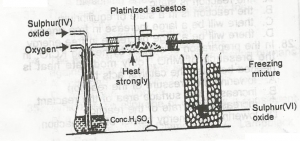
In the diagram above, the purpose of the asbestos to?
absorb impurities
catalyze the reaction
solidify the gas
dry the gas
Correct answer is B
Catalyst like platinize asbestos or vanadium(v)oxide is required to speed up the combination to form sulphur(iv)oxide.
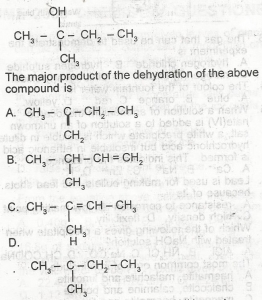
Choose the correct option from the structure above
A
B
C
D
Correct answer is C
No explanation has been provided for this answer.
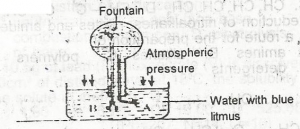
Use the diagram to answer this question.The colour of the fountain water is
blue
orange
red
yellow
Correct answer is C
fountain water has red colour.
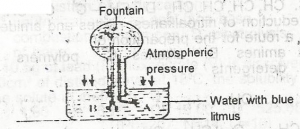
Use the diagram to answer this question. The gas that can be used to demonstrate the experiment is
hydrogen chloride
hydrogen sulphide
nitrogen(ll) oxide
dinitrogen (l) oxide
Correct answer is A
Hydrogen chloride; main reason for using it to demonstrate fountain experiment because of its high solubility in water.
JAMB Subjects
Aptitude Tests