Which of the following is used in rocket fuels?
HNO3
CH3COOH
H2SO4
HCl
Correct answer is A
- Due to its unique powerful oxidizing nature, Nitric acid is often used in rocket fuels as a propeller
NB: Ethanol (not Ethanoic acid) is used alone or mixed with petrol as fuel for racing cars and in rockets.
CO\(^{2-}_{3}\)
Cl-
SO\(_{3}^{2-}\)
NO\(^{2-}_{3}\)
Correct answer is D
NO\(_{3}^{-}\) is confirmed, then the brown ring is due to the formation of FESO\(_4\).NO
it is unaffected
it becomes zero
it decrease
it increase
Correct answer is C
This given equation shows the forward reaction is exothermic, which means an increase in temperature will cause the equilibrium position to shift to left to favor reactant formation, i.e K decreases
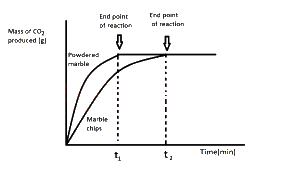
The graph above demonstrate the effect of?
surface area on the rate of reaction
catalyst on the rate of reaction
pressure on the rate reaction
concentration on the rate of reaction
Correct answer is A
No explanation has been provided for this answer.
lowering the pressure of the reaction
increasing the surface area of the reactant
increase the rate of the reaction
lowering the energy barrier of the reaction
Correct answer is C
- The presence of MnO2 increase the rate of the reaction by providing a new reaction path / making more particles possess kinetic energy greater then the activation energy.
JAMB Subjects
Aptitude Tests