X = hydrochloric acid; Y = silver nitrate
X = barium chloride; Y = dilute sulphuric acid
X = hydrochloric acid; Y = sodium hydroxide
X = hydrochloric acid; Y = lead nitrate
X = hydrochloric acid; Y = sodium carbonate
Correct answer is E
No explanation has been provided for this answer.
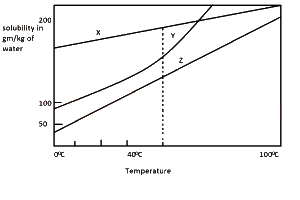
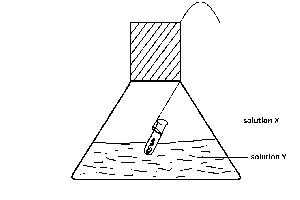
X only
Y only
Z only
X and Z
Y and Z
Correct answer is E
No explanation has been provided for this answer.
basic lead carbonate
lead (II) nitrate
sodium carbonate
zinc nitrate
sodium nitrate
Correct answer is B
Lead nitrate reacts with ammonium hydroxide solution to form a white precipitate. This white precipitate does not dissolve in excess ammonium hydroxide.

T
W
X
Y
Z
Correct answer is D
Y = 19 protons, 19 electrons and 20 neutrons.
Y has electronic configuration 2, 8, 8, 1. Hence, it has one valence electron in the outer shell (Valency of +1).
Mass number = protons + neutrons
= 19 + 20
= 39.
with conc H2SO4 as catalyst
CH3COOH + C2H5OH → CH3COOC2H5 + H2O
esterification
Condensation
saponification
neutralization
Correct answer is A
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests