The impurities formed during the laboratory preparation of chlorine gas are removed by
H2O
NH3
H2SO4
HCl
Correct answer is A
No explanation has been provided for this answer.
M = E/Q
M = EQ
M = Q/E
M = E/2Q
Correct answer is B
No explanation has been provided for this answer.
MnO-4(aq) + Y + 5Fe2+(aq) → Mn2+(aq) + 5Fe2+(aq) + 4H2O(l)
In the equation above, Y is
5H+(aq)
4H+(aq)
10H+(aq)
8H+(aq)
Correct answer is D
No explanation has been provided for this answer.
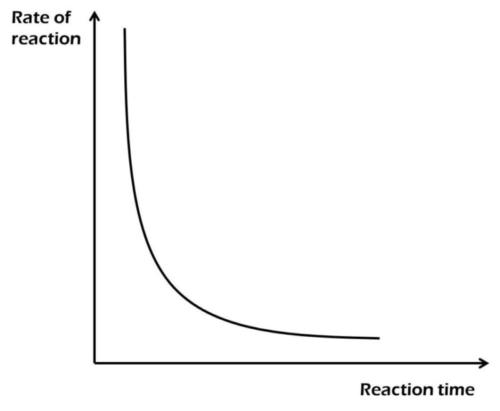
The diagram above best illustrates the effect of decrease in
concentration
temperature
surface area
pressure
Correct answer is A
No explanation has been provided for this answer.
The condition required for corrosion to take place is the presence of
water and carbon (IV) oxide
water, carbon (IV) oxide and oxygen
oxygen and carbon (IV) oxide
water and oxygen
Correct answer is D
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests