large particles
germs
fine particles
odour
Correct answer is C
Potash alum [kAl(S\({O}_4\))\(_2\)] n sodium aluminate (ii) (NaHCO\(_2\)) are added to cause coagulation.
As fine particles to clump together, forming large particles heavy enough to settle at the bottom of the tanks as impurities.
Air boiled out of water as steam is richer in
nitrogen and oxygen
arbon (IV) oxide and oxygen
noble gases and carbon (IV) oxide
oxygen and noble gases
Correct answer is B
Air boiled out of water as steam is richer in carbon(IV)oxide and oxygen.
\( _{11} ^{23}Na + _0^1n → _{11}^{24}Na \)
The reaction above is an example of
nuclear fission
nuclear fusion
artificial transmutation
beta decay
Correct answer is C
Artificial transmutation. It is an artificially induced nuclear reaction caused by the bombardment of a nucleus with subatomic particles or small nuclei. It is also called artififcial radioactivity.
In the periodic table, the electrical and thermal conductivities are properties of elements that
decrease across the period and increase down the group
increase across the period and decrease down the group
decrease across the period and down the group
increase acrosss the period and down the group
Correct answer is A
In the periodic table, electrical and thermal conductivities of elements decrease across the period and increase down the group.
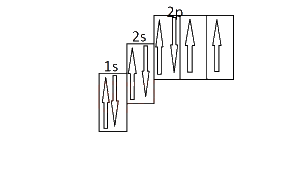
The diagram above represent the electron sub-level for
carbon
nitrogen
oxygen
flourine
Correct answer is C
The number of arrows indicates the number of electrons. Since the atomic number of oxygen is 8, the element is oxygen.
JAMB Subjects
Aptitude Tests