1.650atm
0.825atm
0.413atm
0.275atm
Correct answer is A
Using the combined gas law formula,
Given that P\(_1\) = 0.825, V\(_1\) = 2.76 L, V\(_2\) = 1.38 L, P\(_2\) = ?
And T1 = T2; we would have P\(_2\) = [ P\(_1\) X V\(_1\) ] ÷ V\(_2\)
: [0.825 X 2.76] ÷ 1.38
= 1.650atm
Oxyethene
Oxyhydrocarbon flame
Oxyacetylene flame
Oxymethane flame
Correct answer is C
The reaction of calcium carbide with water, producing acetylene and calcium hydroxide, was discovered by Friedrich Wöhler in 1862. This reaction was the basis of the industrial manufacture of acetylene, and is the major industrial use of calcium carbide.
Acid
Ester of alkanoic acid
Alkali
Alkanol
Correct answer is C
A lye is a metal hydroxide traditionally obtained by leaching wood ashes, or a strong alkali which is highly soluble in water producing caustic basic solutions / an alkaline solution that is used to make soaps. "Lye" most commonly refers to sodium hydroxide (NaOH), but historically has been used for potassium hydroxide (KOH).
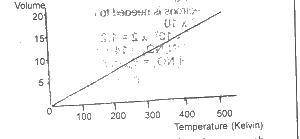
Which of the gas laws does this graph illustrate?
Boyle
Charles
Gay-Lussac
Graham
Correct answer is B
Charles' law describes the effect of temperature changes on the volume of a given mass of gas at a constant pressure.
with cool running water
sodium hydroxide solution
iodine solution
sodium trioxonitrate(v) solution
Correct answer is A
- Sulfuric acid is flushed with a mild, soapy solution if the burns are not severe. Sulfuric acid feels hot when water is added to the acid, but it is better to flush the area and not leave the acid on the skin.
- Don't try to neutralize the burn with acid or alkali. This could cause a chemical reaction that worsens the burn.
JAMB Subjects
Aptitude Tests