The molecular shape and bond angle of water are respectively
linear, 180°
bent, 109.5°
tetrahedral, 109.5°
bent, 105°
Correct answer is D
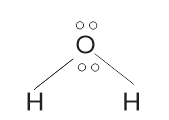
The shape of water molecule = Bent/ V- shaped The bond angle of water = 104.5°/ 105°
ii and iii only
ii and iv only
iii and iv only
i and ii only
Correct answer is B
Ionization energy and electron affinity increase across a period, and decrease down a group.
2.0 moldm\(^{-3}\)
0.25 moldm\(^{-3}\)
1.0 moldm\(^{-3}\)
0.5 moldm\(^{-3}\)
Correct answer is A
Given the Mass of the salt = 58.5g
Volume = 250 cm\(^3\) = 0.25 dm\(^3\)
Mass concentration = \(\frac{Mass}{Volume}\)
= \(\frac{58.5}{0.25}\) = 234 gdm\(^{-3}\)
Solubility (in moldm\(^{-3}\) = \(\frac{234}{111} \)
= 2.11 moldm\(^{-3}\)
\(\approxeq\) 2.0 moldm\(^{-3}\)
Fractional crystallization
Fractional distillation
Filtration
Evaporation
Correct answer is A
No explanation has been provided for this answer.
Which of the following does not support the fact that air is a mixture?
the constituents of air are in a fixed proportion by mass
it cannot be represented with a chemical formula
the constituents of air can be separated by physical by physical means
none of the above
Correct answer is D
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests