I and II only
I and III only
II and III only
I, II and III
Correct answer is D
No explanation has been provided for this answer.
6000A
100A
10A
1A
Correct answer is B
Given Data: Mass of Ag = 36.0g,
Z which is electrochemical equivalent of Ag = 0.0012coulomb
t = 5mins → 5 * 60 = 300secs.
Using M =Z * I * t
make I the subject of the formula
I = \(\frac{M}{Z * t}\) → \(\frac{36}{0.0012 * 300}\)
Current (I) = 100A
The amount of heat needed to raise the temperature of 10kg of Copper by 1K is its
specific heat capacity
latent heat
heat capacity
internal energy
Correct answer is C
The heat capacity of a defined system is the amount of heat (usually expressed in calories, kilocalories, or joules) needed to raise the system's temperature by one degree (usually expressed in Celsius or Kelvin).
fusion reaction
fission reaction
Chain reaction
radioactive decay
Correct answer is A
No explanation has been provided for this answer.
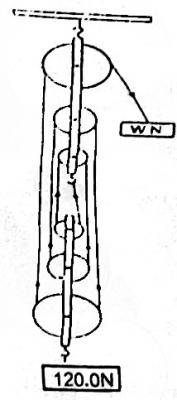
28.0
48.0N
233.0N
50.0N
Correct answer is D
Given Data:
Efficiency = 40% Load = 120N, V.R = 6 (Number of Pulleys)
Efficiency = \(\frac{M.A}{V.R} * 100\)
40 = \(\frac{M.A}{6} * 100\)
6 * 40 = 100 M.A
M.A = \(\frac{240}{100}\) → 2.4
Where M.A = \(\frac{Load}{Effort}\)
2.4 = \(\frac{120}{Effort}\)
: Effort = \(\frac{120}{2.4}\) → 50N
JAMB Subjects
Aptitude Tests