A spontaneous reaction
An exothermic reaction
A non-spontaneous
An endothermic reaction
Correct answer is B
No explanation has been provided for this answer.
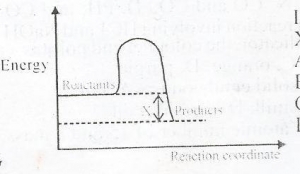
In the diagram above, X is the
Enthalphy
Activated complex
Activation energy
Enthalphy change
Correct answer is D
No explanation has been provided for this answer.
Condensation and hydrolysis
Fermentation and condensation
Polymerization and hydrolysis
Polymerization and condensation
Correct answer is A
No explanation has been provided for this answer.
Geometric isomerism
Positional isomerism
Structural isomerism
Optical isomerism
Correct answer is D
Optical isomers are molecules that are non-superimposable mirror images of each other and they are not identical. Molecules with a chiral carbon show this kind of isomerism. Chiral carbon, also known as asymmetric carbon, refers to carbon atom that are bonded to four different atoms or groups of atoms.
10H+ (aq)
5H+ (aq)
8H+ (aq)
4H+ (aq)
Correct answer is C
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests