An open-tube mercury manometer is used to measure the pre...
An open-tube mercury manometer is used to measure the pressure in a gas tank. When the atmospheric pressure is 101,325 \(P_a\), what is the absolute pressure in \(P_a\) in the tank if the height of the mercury in the open tube is 25 cm higher. density of mercury = \(13600kg/m^3, g = 9.8m/s^2\)
108,986 Pa
165,238 Pa
122,364 Pa
134,645 Pa
Correct answer is D
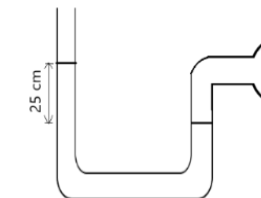
density (ρ) = 13600 \(kgm^{-3}\); g =\(9.8 ms^{-2}\)
\(P_{atm}\) =101,325 Pa; ρ=13600 \(kgm^{-3}\)
Pabs=\(P_{atm}\)+ρgh
⇒Pabs=101,325+13600 x 9.8 x 0.25
⇒Pabs=101,325+33320
⇒ Pabs=134,645Pa
Similar Questions
I. High thermal capacity. II. High sensitivity. III.Easy readability. IV. Accuracy over a wide ra...
A stroboscope can be used to make the wave appear …………. ...
A series RLC circuit is said to resonate, when ...
An electric charge could be transmitted through ...
A ray undergoes a minimum deviation at 40o when it is incident on equilateral triangular glass ...