The IUPAC nomenclature for the structure above is
...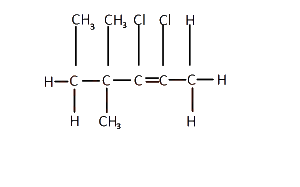
The IUPAC nomenclature for the structure above is
2, 3 - dichloro - 4, 4, 5 - trimethyl pent - 2 - ene
4, 5 - dichloro - 2, 3 - dimethyl hex - 2 - ene
2, 3 - dichloro - 4, 4 - dimethyl hex - 2- ene
2, 3 dichloro - 2, 2 - dimethyl hex - 2 - ene
Correct answer is C
The longest carbon chain in the compound is the hex-2-ene.
Counting all the attachments, the IUPAC nomenclature of the compound is 2,3 - dichloro-4,4- dimethyl hex-2-ene.
Similar Questions
The process of extraction of iron from its ore is ...
In the extraction of aluminum from purified bauxite by electrolysis, cryolite is used because ...
Which of the following sketches is a graphical representation of Boyle's law? ...
What is the chemical symbol for Berkelium? ...
The gas that will form a white precipitate with acidified silver trioxonitrate (v) is ...
Which of the following statements about a molar solution is correct? it ...
The number of isomers that can be obtained from C4H10 is...
Solubility curve can be used in the determination of the ...