From the diagram above, find the amount of solute deposit...
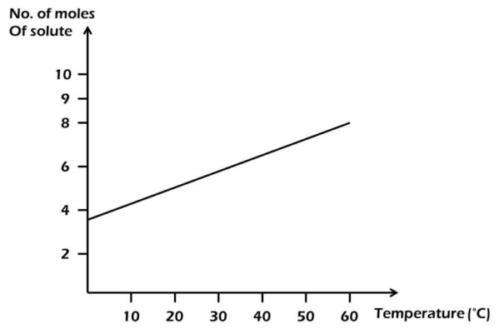
From the diagram above, find the amount of solute deposited when 200 cm33 of the solution is cooled from 55°C to 40°C.
0.10 mole
0.20mole
0.01 mole
0.02 mole
Correct answer is B
From the diagram,
55°C = 7 moles; 40°C = 6 moles.
Amount of solute deposited = 7 - 6 = 1 mole.
1000 cm\(^3\) = 1 mole
200 cm\(^3\) = x
x = \(\frac{200 \times 1}{1000}\)
= 0.20 mole.
Similar Questions
A mixture of kerosene and diesel oil can be separated by ...
Which of the following statements is not correct?...
What is the chemical symbol for Lanthanum? ...
Which of the following represents an aromatic compound? ...
Which of the following properties is characteristic of the halogens? ...
An example of a polysaccharides is? ...
Amino acids are obtained from proteins by ...
NH3(g) + HCl(s) → NH 4Cl (s) The entropy change in the system above is...