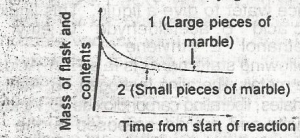
The following graph demonstrates the rate of reaction between calcium carbonate (marble)and dilute hydrochloric acid. The graph shows that the rate of reaction is initially greatest when
the initial slope of the curve is steepest and this occur when small pieces of marble are used and is due to a small surface area of reactant
the occur when large pieces of marble are used and is due to a small surface area of reactant
the initial slope of the curve is steepest and this occurs when small pieces of marble are used and is due to a large surface area of reactant
the initial slope of the curve is steepest and this occur when large surface area of reactant
the initial slope of the curve is least steep and this occur when small pieces of marble are used and is due to the increased surface area of the reactant
Correct answer is C
No explanation has been provided for this answer.
Similar Questions
The following oxides react with water except ...
The oxidation state of oxygen in tetraoxosulphate (VI) acid is ...
The most common method of preparing insoluble salts is by ...
Copper can best be purified by ...
Which of the following is a property of ionic chlorides? ...
Which of the following compounds has the lowest boiling point? ...