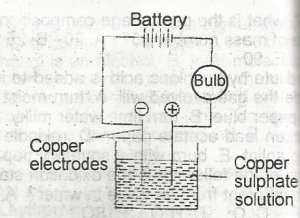
Copper sulphate solution was electrolysed using the apparatus show above. Which of the following changes are observed?
The bulb lights and copper is deposited at both electrodes
the bulb lights and copper is deposited at the anode and disappears from the cathode
the bulb lights and copper is deposited at the cathode and oxygen liberated at the anode
the bulb lights and copper is deposited at the cathode and disappears from the anode
the bulb lights but no additional changes are observed
Correct answer is D
No explanation has been provided for this answer.
Similar Questions
Addition of an aqueous solution of a salt gives a white precipitate. The salt is likely to be a? ...
When excess ethanol is heated to 145oC in the presence of concentrated H2SO4, the product is...
What is the product of the reaction between propene and 1 mole of hydrogen iodide? ...
A major effect of oil pollution in coastal waters is the? ...
Reduction of alkanones with LialH4 produces ...
In the diagram above, the mixture of the solids P and Q can be separated by? ...
The condensation of several amino acid molecules gives ...
An element which can exist in two or more forms in the same physical state, exhibits ...
Which of the following statements about enthalpy of neutralization is correct? It ...