The solubility curve of a solid X (molecular mass = 160) is ...
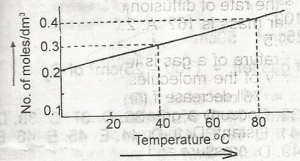
The solubility curve of a solid X (molecular mass = 160) is as show above. 1,000 cm3 of a saturated solution of X at 80°C is cooled to 40°C . The weight of X crystalized out would be
A.
10 g
B.
12 g
C.
14 g
D.
16 g
E.
18 g
Correct answer is D
At 80°C, we have \(1000cm^{3} = 0.4mol/cm^{3}\)
40°C = \(0.3mol/cm^{3}\)
hence, the loss in concentration = \(0.1mol/cm^{3}\)
= \(0.1 \times 160 = 16g\)
Similar Questions
Which of the following is NOT correct test for the named organic compound in each case? ...
The nucleus of a hydrogen atom consists of ...
The group IA metals are not found free in nature because they ...
The number of isomers formed by C<sub>6</sub>H<sub>14</sub> is ...
Which of the following statements is FALSE? ...
When air is passed through potash and then pyrogallol, the components are noble gases ...
Which of the following compounds is a base? ...
A gas that turns a filter paper previously soaked in lead ethanoate solution black is? ...