Which of the curves shown in the figure given represents the...
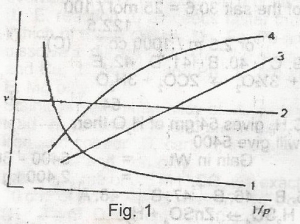
Which of the curves shown in the figure given represents the relationships between the volume(V) and pressure(P) of an ideal gas at constant temperature
A.
1
B.
2
C.
3
D.
4
E.
1 and 3
Correct answer is C
No explanation has been provided for this answer.
Similar Questions
Boyle's law can be expressed mathematically as ...
What volume of 1.5M solution of KOH would contain 0.045 moles ...
The green colour solution of an Fe2 salt changes to a brown solution of an Fe3+ salt by ...
Which of the following raw materials is used in the plastic industry ? ...
Copper (ll) tetraoxosulphate (VI) is often added to swimming pools because it...