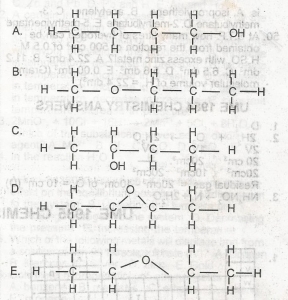
Use the diagram above to answer this question Which of the following structural formula is NOT isomeric with the others?
A
B
C
D
E
Correct answer is D
Isomers have the same molecular structures(but different structures). so whatever the structures are, they must contain the same amount of carbon, the same amount of hydrogen, and the same amount of oxygen as in this case. Just count the number of hydrogens (including the hydrogen in OH). you should get 10 hydrogens from A, B, C, and E. in total, you should have C4H10O. options A, B, C, and E have this in common. but the option D has only C4H8O.
Similar Questions
When element 20A combines with element 8Y,...
When ΔH is negative, a reaction is said to be ...
Which of the following bonds exist in crystalline ammonium chloride (NH4Cl)?...
Chlorine is used in water purification because it ...
Which of the following contains two amphoteric oxides? ...
Which of the following relationships correctly expresses the Boyle's law? ...
What volume of oxygen will remain after reacting 8cm\(^3\) of hydrogen gas with 20cm\(^3\) of o...