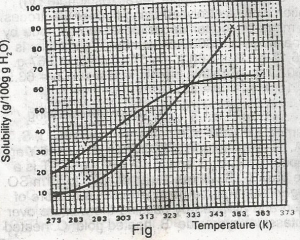
The diagram shown above represents the solubility curves of two salts, X and Y, in water, use this diagram to answer the question. If 80 g each of X and Y are taken up in 100 g of water at 353 K we shall have
only 10g of X undissolved
only 16 g of Y undissolved
10 g of X and 16 g of Y undissolved
all X and Y dissolved
all X and Y undissolved
Correct answer is B
For salt X, from the graph, 80g of X dissolves by 349K, so by 353K, all 80g of X is dissolved totally in 100g of water. For salt Y, from the graph, 64g of Y dissolves by 353K, so (80-64)g of Y is left undissolved. This is 16g of Y left undissolved at that temperature.
Similar Questions
The atomic number of an atom would be equal to its mass number if it ...
The boiling point of water is higher than that of methanol because? ...
A measure of the degree of disorderliness in a chemical system is known as the ...
3H\(_{2(g)}\)+ N\(_{2(g)}\)⇔ 2NH\(_{3(g)}\) ; H= -ve In the reaction above, lowering of temper...
CH4(g) + Cl2(g) → CH3 Cl(s) + HCl(g) The major factor that influence the rate of the reaction abo...
The air pollutant unknown in nature is...
Which of the following salts is not prepared by precipitation? ...
f 5.0 cm\(^-3\) of 0.200 mol dm(^-3\) Na\(_2\)CO\(_3\) was diluted to 250cm(^-3\)&nbs...