The diagram shown above represents the solubility curves of ...
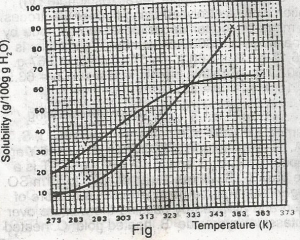
The diagram shown above represents the solubility curves of two salts, X and Y, in water, use this diagram to answer the question. If the molar mass of X is 36 g, the number of moles of X dissolved at 343 K is
A.
0.2 moles
B.
0.7 moles
C.
1.5 moles
D.
2.0 moles
E.
3.0 moles
Correct answer is D
The molar mass of x = 36g. At 343k, 72g of x dissolved
Molarity of x = \(\frac{72}{36}\) = 2.00moles.
Similar Questions
Which of the following salts is stable to heat? ...
The alkaline hydrolysis of fats and oils produces soap and...
The following glasswares are used to measure the volume of liquids EXCEPT ...
Which of the following equations show that a reaction is in equilibrium? ...
The entropy and enthalpy of a system are a measure of ...
Which of the following represent hybridization in ethyne? ...