In the experiment above. Z can be
...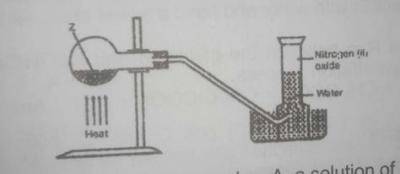
In the experiment above. Z can be
a solution of sodium dioxonitrate (lll) and ammonium chloride
a solution of lead trioxnitrate (V)
a solution of sodium trioxonitrate (V) and ammonium chloride
concentrated tetraoxosulphate (VI) acid and sodium trioxonitrate (V)
Correct answer is C
Nitrogen(I) oxide(laughing gas) can be prepared in the laboratory by the action of heat on a mixture of sodium trioxonitrate (V) and ammonium chloride, this colourless gas is collected over water.
Similar Questions
What is the atomic number of Chlorine? ...
The reaction of vegetable oil with a solution of wood ash is ...
The quality of electricity required to discharge 1 mole of univalent ion is ...
Aluminium hydroxide is used in the dyeing industry as a ...
What is the chemical symbol for Lanthanum? ...
Iron galvanized with zinc is cathodically protected from corrosion. This is because? ...
The mass number of an atom is? ...
Which of the following pairs cannot be represented with a chemical formula? ...