In the diagram above, curve X represents the energy profi...
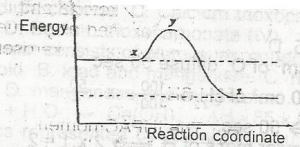
In the diagram above, curve X represents the energy profile for a homogeneous gaseous reaction. Which of the following conditions would produce curve Y for the same reaction?
Increase in temperature
Increase in the concentration of a reactant
Addition of a catalyst
Increase in pressure
Correct answer is C
No explanation has been provided for this answer.
Similar Questions
The reddish–brown rust on iron roofing sheets consists of ...
Particles in a solid exibit ...
The compound formed between 14X and 16Y is _______?...
What number of moles of oxygen would exert a pressure of 10 atm at 320 K in an 8.2 dm\(^{2}\) c...
Which of the following is not decomposed by heat?...
A secondary alkanol can be oxidized to give an? ...