The ∆H for the reaction represented by the energy profi...
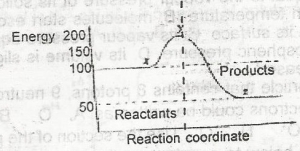
The ∆H for the reaction represented by the energy profile above is
A.
-100 kJ mol--1
B.
+ 100 kJ mol-1
C.
+50 kJ mol-1
D.
-50 kJ mol-1
Correct answer is D
No explanation has been provided for this answer.
Similar Questions
The IUPAC name of C2H6COOC2,/sub>H6 is...
Which of the following pairs of solutions will produce a precipitate when mixed? ...
The condition required for corrosion to take place is the presence of ...
Which of the following halogens is liquid at room temperature? ...
When steam is passed through red hot carbon, which of the following are produced? ...
Alkanals can be differentiated from alkanones by reaction with? ...