2HCL(ag) + CaCO3(ag) → CaCL2(ag) + H2O(1) + CO from ...
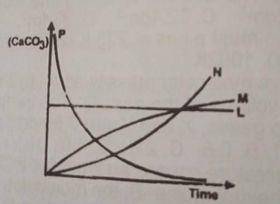
2HCL(ag) + CaCO3(ag) → CaCL2(ag) + H2O(1) + CO from the reaction above, which of the following curves represents the consumption of calcium trioxocarbonate (IV) as dilute HCL is added to it?
L
M
N
P
Correct answer is D
The concentration of the \(CaCO_3\) decreases as reaction time decreases. therefore option D is correct.
Similar Questions
Which of the elements in the table below would react more readily with chlorine? &nbs...
An example of aliphatic unsaturated hydrocarbon is ...
The IUPAC name for the compound is...
Which of the following equations does not illustrate correctly one of the reactions of chlorine? ...
Which of the following chlorides is insoluble in water? ...
Which of the following compounds is the best possible anti-knock agent for petrol? ...
Which of the following pairs of reagents reat to produce hydrogen? ...
Which of the following compounds is used as a gaseous fuel? ...