The option above shows the PH changes for the titration o...
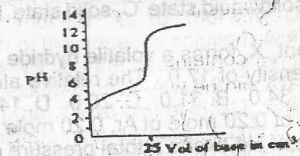
The option above shows the PH changes for the titration of a
strong acid versus strong base
weak acid versus strong base
strong acid versus weak base
weak acid versus weak base
Correct answer is B
Option B is a graph of weak a acid and strong base
Similar Questions
The Van der waals' forces are dominant intermolecular forces in_______? ...
Which of the following are structural isomers? ...
What is the atomic number of Rubidium? ...
Which of the following compounds is an example of an electrovalent bond? ...
A fixed mass of gas occupies 92cm3 at 3°C. What will be its volume at 18C if...
Which of the following is a property of ionic chlorides? ...
In the laboratory preparation of ammonia, the flask is placed in a slanting position so as to ...
The bleaching action of chlorine gas is effective due to the presence of ...