Use the graph above to answer this question. A sample, X,...
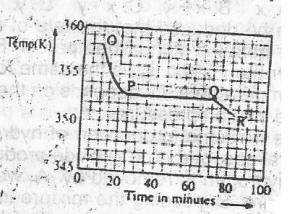
Use the graph above to answer this question. A sample, X, solid at room temperature, was melted, heated to a temperature of 358 K and allowed to cool as shown in OPQR. The section PQ indicates that X is
A.
a mixture of salts
B.
a hydrated salt
C.
an ionic salt
D.
a pure compound
Correct answer is D
No explanation has been provided for this answer.
Similar Questions
The metal extracted from cassiterite is ...
The stability of the noble gases is due to the fact that they? ...
What is the chemical symbol for Xenon? ...
Hydrogen is used in oxy-hydrogen flames for melting metals because it ...
Which of the following transition metals is not attracted to external magnetic field? ...