Use the graph above to answer this question. A sample, X,...
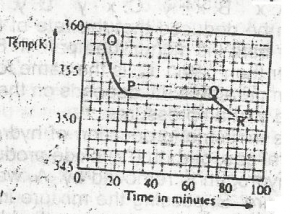
Use the graph above to answer this question. A sample, X, solid at room temperature, was melted, heated to a temperature of 358 K and allowed to cool as shown in OPQR.The section OP suggests that X is in the
liquid sate
solid/liquid state
solid state
gaseous state
Correct answer is A
No explanation has been provided for this answer.
Similar Questions
Which of the following compounds of trioxonitrate (V) will decompose to give dinitrogen (i) oxi...
When a bottle of Coca-Cola is opened, bubbles of evolve. The gas is? ...
When steam is passed over red hot carbon the substances produced are ...
(I). 3CuO(s) + 2NH3(g) -----> 3Cu(s) + 3H2O(l) + N2(g) (II). 2NH3(g)&n...
NH3(g) + HCl(s) → NH 4Cl (s) The entropy change in the system above is...
Which of the following metallic oxides is amphoteric? ...
A substance is said to be impure if ...
What is the IUPAC name for the following compound? HC\(\equiv\)CCH\(_{3}\)...