In the experiment above, X could be a solution of
...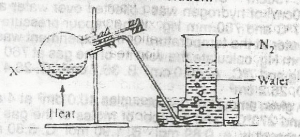
In the experiment above, X could be a solution of
sodium trioxonitrate (V) and ammonium chloride
sodium trioxonitrate (lll) and ammonium chloride
lead (ll) trioxonitrate (V) and copper turnings
potassium trioxonitrate (V) and copper turnings
Correct answer is B
Nitrogen is prepared in the laboratory by heating an equimolar aqueous solution of ammonium chloride and sodium nitrite. The ammonium nitrite formed as a result of a double decomposition reaction, decomposes to form nitrogen gas.
\(NH_4Cl_{(aq)}+NaNO_{2(aq)} → NH_4NO_{2(aq)}+NaCl_{(aq)}\)
\(NH_4NO_{2(aq)} →N_{2(g)} +2H_2O_{(l)}\).
(source: understanding chemistry by G O Ojukuku)
Similar Questions
Which of the following factors would affect the equilibrium constant? ...
Consider the neutralization reaction represented by the following equation: Na2CO3 + 2HNO 3 → 2...
Which of the following are made by the process of polymerization? ...
Cathodic protection of metals is based on ...
Cu2S(g) + O2(g) → 2Cu + SO2(g) What is the change in the oxidation number of copper in the rea...
A consequence of global warming is? ...
In the diagram above, gas X is ...
Which of the following factors would not affect the solubility of a gas? ...