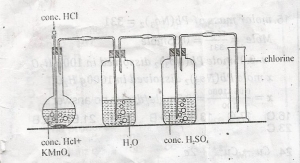
In the diagram, the function of the concentrated H2SO4 is to?
purify the gas
dry the gas
liquefty the gas
remove odour
Correct answer is B
Concentrated sulphuric acid is a very effective drying agent. The chlorine obtained as the product from the above reaction contains traces of water vapour, and hydrogen chloride (HCl) vapours. HCl vapours are removed by passing the gas through water, and the traces of water vapour are removed by passing through conc.sulphuric acid.
Similar Questions
The enzyme that catalyses the conversion of glucose te ethanol and carbon (IV) oxide is ...
The molar enthalpy change accompanying the removal of an electron from a gas phase atom or ion ...
What is the atomic number of Gold? ...
Which of the following gives a precipitation when treated with NaOH Solution? ...
What is the atomic number of Neon? ...
Insoluble salts can be prepared by? ...
Fluorine does not occur in the free state in nature because ...
Which of the following is not a property of magnesium oxide? ...