The electron configuration of \(_{26}Fe^{3+}\) is?
...The electron configuration of \(_{26}Fe^{3+}\) is?
[Ar]4s23d6
[AR]4s23d3
[AR]4s13d4
[AR]4s03d5
Correct answer is D
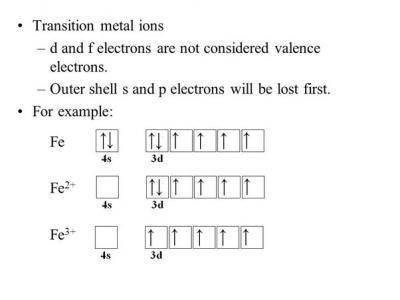
The neutral Iron atom has the electric configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d6.to write the electronic config. of Fe3+ we have to subtract 3 electrons from the outermost shell which is 4s and 3d orbitals. Thus we obtain the electronic configuration for Fe3+ as:
1s2 2s2 2p6 3s2 3p6 4s0 3d5
OR
[AR] 4s0 3d5.
Similar Questions
The property which makes alcohol soluble in water is the ...
The most volatile fraction obtained from fractional distillation of crude petroleum contains? ...
The bond formed when ammonia reacts with hydrogen ion to form ammonium ion is ...
The synthetic detergents are preferred to soap for laundry using hard water because? ...