The curve depicts titration between a strong acid of pH
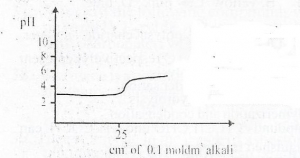
The curve depicts titration between a strong acid of pH
Strong acid and strong base
Strong acid and weak base
Weak acid and weak base
Weak acid and strong base
Correct answer is B
No explanation has been provided for this answer.
Similar Questions
Determine the volume of 0.100 mol of HCl in \(0.250 mol dm^3\) of solution....
If glucose is heated with concentrated tetraoxosulphate(vi)acid, it will be dehydrated to?...
Chlorine is produced commmercially by? ...
Which of the following equimolar solutions would have the highest conductivity? ...
When ethanol is heated with excess concentrated sulphuric acid, the ethanol is? ...
The bond which joins two ethanoic acid molecules in the liquid state is...