In the shown experiment (Fig. 1) the litmus paper will in...
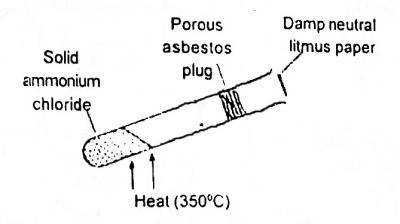
In the shown experiment (Fig. 1) the litmus paper will initially
be bleached
turn green
turn red
turn blue
Correct answer is D
\(NH_4Cl → NH_3 + HCl\). rate of diffusion of substances depends too on their molar mass, the smaller the molar mass of a substance the faster the rate of diffusion of that substance. so the litmus paper initially turns blue because ammonia will get to it first since it diffuses faster than HCl.
Similar Questions
Oxygen gas can be prepared by heating? ...
The molecule which has the highest percentage of ionic character among the following is ...
An organic compound which decolorizes bromine water is likely to be? ...
When element \(_{20}\)Y combines with element \(_8\)Z, it forms...
What is the trend for ionization energy across a period in the periodic table? ...
The impurities formed during the laboratory preparation of chlorine gas are removed by ...
What weight of NaCl is needed to make 2.0 dm3 of a 1.5M solution? [Na = 23, Cl = 35.5]...