The diagram above illustrates a conical flask containing ...
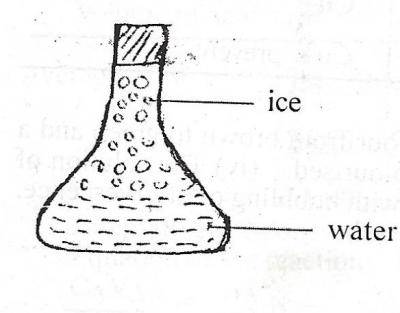
The diagram above illustrates a conical flask containing water and ice. Which of the following is correct about the diagram?
The water is at lower temperature than the ice
Energy is absorbed when the ice changes to water
Energy is released when the ice changes to water
The water molecules vibrate about a fixed point
Correct answer is B
Heat energy near the ice gets absorbed into the ice. The energy breaks the bonds of the ice, causing it to melt into liquid water. On other words, when ice melts, the energy gets absorbed into the water.
Similar Questions
Zinc displaces copper from an aqueous solution of copper (ll) salt because ...
How much sodium hydroxide is required to make 250 cm3 of 0.1 molar solution? Na=23; 0=16; H=1] ...
If the volume of a given mass of gas at 298 K and pressure of 205.2 x 10\(^3\) Nm\(^{-2}\) ...
Which of the following oxides of nitrogen has oxidation number of + 1? ...
The products of the electrolysis of dilute sodium chlorid solution with platinum electrodes are ...