A primary alkanol has a molecular mass of 60. What is the...
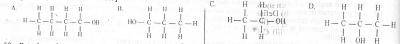
A primary alkanol has a molecular mass of 60. What is the structural formula of the compound? [C= 12.0, H= 1.0, O= 16.0)
A
B
C
D
Correct answer is B
Alkanols have the formula \(C_{n}H_{2n+1}OH\).
⇒ \( 12n + (1\times (2n+1)) + 16.0 + 1.0 = 60\)
= (14n +18 = 60\)
\(n = \frac{60-18}{14} = \frac{42}{14} = 3\)
Hence, the alkanol is gotten by putting n=3,
=\(C_{3}H_{7}OH\)
Similar Questions
The nucleus of a hydrogen atom consists of ...
The following statements are correct except ...
The gas responsible for most of the fatal explosions in coal mines is...
A suitable reagent for distinguish between ethanoic and ethanol is ...
Detergents are manufactured with straight hydrocarbon chains, is to make them ...
The reaction C2H6 → C2H + H2 is known as...
A spot of oil paint on a shirt can best be removed using? ...
In which of the following are water molecules in the most disorderly arrangement? ...
Which of the following constitutes an advantage in the use of hard water? ...