During the nuclear reaction described by
| 235 | W → | 235 | X→ | 231 | Y |
| 92 | 93 | 91 |
the particles emitted are respectively
A.
α and α
B.
α and β
C.
β and β
D.
β and α
Correct answer is D
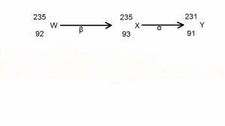
The decay process illustrated above shows that the lost β -particle to decay to the X by increase its atomic number ( from 92 to 93) and leaving the mass no. unaffected. There after, x decayed by -α emission to give Y, by decreasing the mass no. by 2 ( from 93 to 91).
Thus , the emission is β and α particles respectively
Similar Questions
Which of the following is/are longitudinal wave(s)? I. Light waves from the sun II. Sound waves...
Which of the arrangements of radiations below shows decreasing order of wavelengths? ...
If a wire 30 cm long is extended to 30.5 cm by a force of 300 N. find the strain energy of wire...
the property that is propagated in a traveling wave is ...