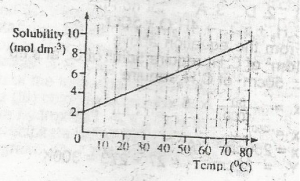
117.00 g
58.50 g
11.70 g
5.85 g
Correct answer is A
No explanation has been provided for this answer.
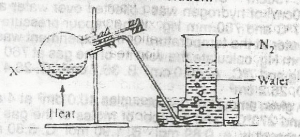
In the experiment above, X could be a solution of
sodium trioxonitrate (V) and ammonium chloride
sodium trioxonitrate (lll) and ammonium chloride
lead (ll) trioxonitrate (V) and copper turnings
potassium trioxonitrate (V) and copper turnings
Correct answer is B
Nitrogen is prepared in the laboratory by heating an equimolar aqueous solution of ammonium chloride and sodium nitrite. The ammonium nitrite formed as a result of a double decomposition reaction, decomposes to form nitrogen gas.
\(NH_4Cl_{(aq)}+NaNO_{2(aq)} → NH_4NO_{2(aq)}+NaCl_{(aq)}\)
\(NH_4NO_{2(aq)} →N_{2(g)} +2H_2O_{(l)}\).
(source: understanding chemistry by G O Ojukuku)
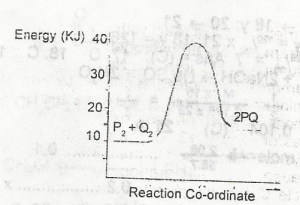
In the diagram above , the activation energy for the backward reaction is
+5kJ
+15KJ
+25KJ
+30KJ
Correct answer is C
For the backward reaction, the activation energy = 40kJ - 15kJ = +25kJ

The diagram above represents an atom that can combine with chlorine to form
a covalent bond
an electrovalent bond
a hydrogen bond
a co-ordinate bond
Correct answer is B
No explanation has been provided for this answer.
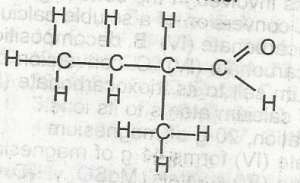
The compound above is the product of the oxidation of
2-methlbutan-2-ol
2-methylbutan -1- ol
2, 3-dimethylprop 1-ol
Pentan -2- ol
Correct answer is B
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests