Which of the following is an item in the debit side of the sales ledger control account?
Interest charged by suppliers
Bills receivable honored
Transfer of credit balances from credit control account
Transfer of debit balance from creditors control account
Correct answer is A
Recall sales ledger control account are used to track trade debtors, that is goods sold on credit. We already know that we debit all income and credit expenses. The debit side of the account contains interest charged by suppliers because the goods were sold on credit and its considered an income.
Acid
Ester of alkanoic acid
Alkali
Alkanol
Correct answer is C
A lye is a metal hydroxide traditionally obtained by leaching wood ashes, or a strong alkali which is highly soluble in water producing caustic basic solutions / an alkaline solution that is used to make soaps. "Lye" most commonly refers to sodium hydroxide (NaOH), but historically has been used for potassium hydroxide (KOH).
Which of the following is a debit item in the purchases ledger control account?
Balance b/d
Cheque dishonoured
Purchases
Balance c/d
Correct answer is D
No explanation has been provided for this answer.
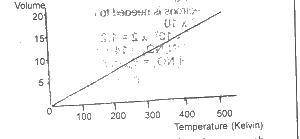
Which of the gas laws does this graph illustrate?
Boyle
Charles
Gay-Lussac
Graham
Correct answer is B
Charles' law describes the effect of temperature changes on the volume of a given mass of gas at a constant pressure.
with cool running water
sodium hydroxide solution
iodine solution
sodium trioxonitrate(v) solution
Correct answer is A
- Sulfuric acid is flushed with a mild, soapy solution if the burns are not severe. Sulfuric acid feels hot when water is added to the acid, but it is better to flush the area and not leave the acid on the skin.
- Don't try to neutralize the burn with acid or alkali. This could cause a chemical reaction that worsens the burn.
JAMB Subjects
Aptitude Tests