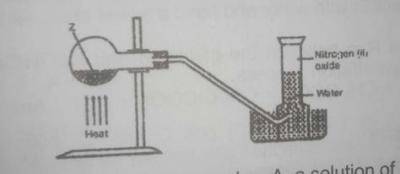
In the experiment above. Z can be
a solution of sodium dioxonitrate (lll) and ammonium chloride
a solution of lead trioxnitrate (V)
a solution of sodium trioxonitrate (V) and ammonium chloride
concentrated tetraoxosulphate (VI) acid and sodium trioxonitrate (V)
Correct answer is C
Nitrogen(I) oxide(laughing gas) can be prepared in the laboratory by the action of heat on a mixture of sodium trioxonitrate (V) and ammonium chloride, this colourless gas is collected over water.
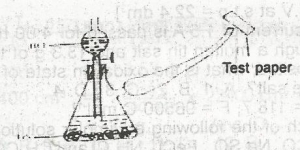
The appropriate test paper to use in the above experiment is moist
litmus paper
potassium heptaoxodichromate (IV) paper
lead (ll) trioxonitrate (V) paper
universal indicator paper
Correct answer is C
No explanation has been provided for this answer.

two
three
four
five
Correct answer is C
3.0 x 10-5 x 4 = 1.2 x 10-4
.: The rate increase by a factor of 4
Sulphur (IV) oxide and hydrogen chloride.
Carbon (IV) oxide and ammonia
Ammonia and hydrogen chloride
Carbon (IV) oxide and Sulphur (IV) oxide
Correct answer is C
No explanation has been provided for this answer.

ionic
covalent
dative
metallic
Correct answer is B
Element Y is Silicon
Element Z is Chlorine
Silicon reacts with chlorine to form the covalent compound SiCl4
JAMB Subjects
Aptitude Tests