0.10 mole
0.20mole
0.01 mole
0.02 mole
Correct answer is B
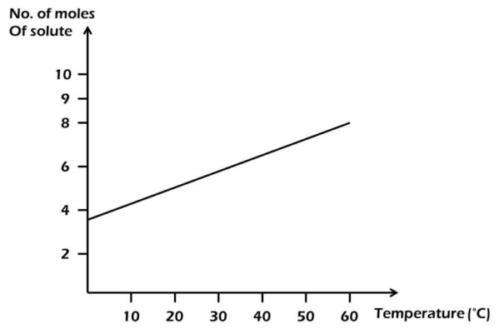
From the diagram,
55°C = 7 moles; 40°C = 6 moles.
Amount of solute deposited = 7 - 6 = 1 mole.
1000 cm\(^3\) = 1 mole
200 cm\(^3\) = x
x = \(\frac{200 \times 1}{1000}\)
= 0.20 mole.
The arrangement of particles in crystal lattices can be studied using
X - rays
γ - rays
α - rays
β - rays
Correct answer is A
No explanation has been provided for this answer.
The molecular lattice of iodine is held together by
dative bond
metallic bond
hydrogen bond
van der Waal's forces
Correct answer is D
No explanation has been provided for this answer.
An isotope has an atomic number of 15 and a mass number of 31. The number of protons it contain is
16
15
46
31
Correct answer is B
No explanation has been provided for this answer.
6.9
7.1
6.2
6.8
Correct answer is A
R.A.M = m\(_1 \alpha_1\) + m\(_2 \alpha_2\)
= 7(90/100) + 6(10/100)
= 6.3 + 0.6
= 6.9
JAMB Subjects
Aptitude Tests