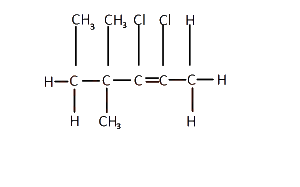
The IUPAC nomenclature for the structure above is
2, 3 - dichloro - 4, 4, 5 - trimethyl pent - 2 - ene
4, 5 - dichloro - 2, 3 - dimethyl hex - 2 - ene
2, 3 - dichloro - 4, 4 - dimethyl hex - 2- ene
2, 3 dichloro - 2, 2 - dimethyl hex - 2 - ene
Correct answer is C
The longest carbon chain in the compound is the hex-2-ene.
Counting all the attachments, the IUPAC nomenclature of the compound is 2,3 - dichloro-4,4- dimethyl hex-2-ene.
If glucose is heated with concentration tetraoxosulphate (VI) acid, it will be dehydrated to
carbon
carbon (IV) oxide
ethene
ethanol
Correct answer is A
If glucose is heated with concentrated tetraoxosulphate(VI)acid, it will be dehydrated to carbon.
If the silver mirror test is positive, it indicates the presence of an
alkyne
alkanol
alkanone
alkanal
Correct answer is D
Alkanals give a positive result for the silver mirror test(Tollen's reagent).
The type of isomerism shown by cis- and trans- isomers is
optical isomerism
positional isomerism
functional isomerism
geometrical isomerism
Correct answer is D
The type of isomerism shown by the cis- and trans- isomers is geometrical isomerism
The most common ores of iron include
haematite, malachite and limonite
chalcocite, calamine and bornite
magnetite, haematite and limonite
magnetite, chalcocite and bornite
Correct answer is C
The most common ores of iron are magnetic, haematite and limonite
JAMB Subjects
Aptitude Tests