Extractive distillation
Filtration followed by distillation
Neutralization with sodium hydroxide followed by distillation
Neutralization with sodium hydroxide followed by filtration
Correct answer is A
Due to the presence of maximum Azeotrope,we use extractive distilination (with extractive distilation agents like sulfone) used for mixtures with low relative volatility,nearing unity.
Azeotrope is a mixture of liquids that has a constant boiling point because the vapour has thesame composition as the mixture.
lowering the pressure of the reaction
increasing the surface area of the reaction
increasing the rate of the reaction
increasing the energy barrier of the reaction
Correct answer is C
No explanation has been provided for this answer.
Which of the following pairs of substances will react further with oxygen to form a higher oxide?
CO\(_2\) and H\(_2\)O
NO and H\(_2\)O
CO and CO\(_2\)
SO\(_2\) and NO
Correct answer is D
No explanation has been provided for this answer.
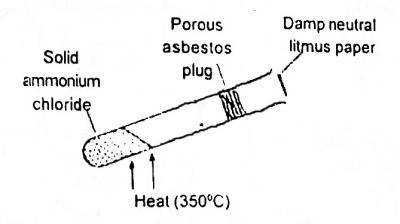
In the shown experiment (Fig. 1) the litmus paper will initially
be bleached
turn green
turn red
turn blue
Correct answer is D
\(NH_4Cl → NH_3 + HCl\). rate of diffusion of substances depends too on their molar mass, the smaller the molar mass of a substance the faster the rate of diffusion of that substance. so the litmus paper initially turns blue because ammonia will get to it first since it diffuses faster than HCl.
2.2M
1.1M
0.2M
0.11M
Correct answer is C
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests