The following atoms of carbon 126C, 136C and 146C can be described as?
Allotropes
Isomers
Isotopes
Isotones
Correct answer is C
No explanation has been provided for this answer.
Increase the reactant production
Increase the value of the equilibrium constant
Shift the equilibrium to the left
Decrease the value of the equilibrium constant
Correct answer is B
No explanation has been provided for this answer.
Concentration
Catalyst
Temperature
Light
Correct answer is D
No explanation has been provided for this answer.
From the diagram above, an ideal gas can be represented by
K
M
L
N
Correct answer is D
see diagram in textbook
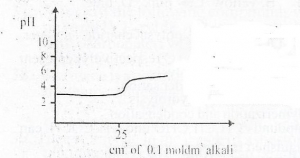
The curve depicts titration between a strong acid of pH
Strong acid and strong base
Strong acid and weak base
Weak acid and weak base
Weak acid and strong base
Correct answer is B
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests