Which of the following compounds is the least soluble in water?
CaCl2
CaSO4
NaCl
Na2SO4
Correct answer is B
No explanation has been provided for this answer.
Solubility is practically applied in
Fractional distillation
The determination of pH
The determination of saturation in hydrocarbons
Solvent extraction
Correct answer is D
No explanation has been provided for this answer.
Which of the following oxides can be reduced by hydrogen?
Aluminium oxide
Magnesium oxide
Sodium oxide
Silver oxide
Correct answer is D
Considering the electrochemical series, we can say that hydrogen can reduce only oxides of copper, mercury, silver, and gold as these metals are less reactive than hydrogen. The reduction of oxides of tin, iron, zinc, magnesium, calcium, and potassium is not possible by hydrogen as these metals are more reactive than hydrogen
CH3COOONa
Mg(OH)Cl
NaHSO4
(NH4)2SO4
Correct answer is C
Acidic Salt: A normal salt which is formed by the neutralization of a strong acid and weak base is called acidic salt
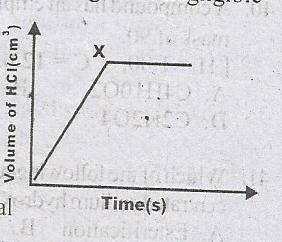
The reaction was slowed down
All the dilute HCl has reacted
All the Mg has reacted
Hydrogen gas is produced at a steady rate
Correct answer is C
No explanation has been provided for this answer.
WAEC Subjects
Aptitude Tests