Which of the following processes is an endothermic reaction?
Dissolving NH4CI crystals in water
Addition of concentrated H2SO4
Dissolving NaOH pellets in water
Passing SO3 gas into water
Correct answer is A
No explanation has been provided for this answer.
formation
hydration
neutralization
solution
Correct answer is C
No explanation has been provided for this answer.
1.12dm3
2.24dm3
5.6dm3
56.0dm3
Correct answer is C
2NaHCO3(s) \(\rightarrow\) 2Na2CO 3(S) + CO 2(g) + H2O(g)
2 moles of 2NaHCO3(s) produces 22.4dm3 of CO2
0.5 moles of 2NaHCO3 will produce \(\frac{0.5 \times 22.4}{2}\)
= \(\frac{11.2}{2}\)
= 5.6 dm3
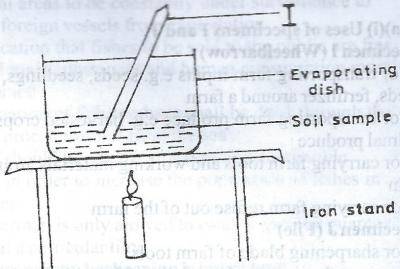
The aim of the experiment illustrated above is to
Determine soil texture
Ascertain water content of the soil
Examine the structure of the soil
Determine soil acidity
Correct answer is B
No explanation has been provided for this answer.
0.10mol dm-3
0.20mol dm-3
0.40mol dm-3
2.00mol dm-3
Correct answer is C
\(V_1\) = 80cm3, \(C_1\) = 0.5mol/dm3
\(C_2\) = ? \(V_2) = 20cm3 + 80cm3 = 100cm3
using the dilution formula, \(C_1 V_1 = C_2 V_2\)
0.5 x 80 = \(C_2\) x 100
\(C_2 = \frac{ 40}{100}\) = 0.4mol\(dm^{-3}\)
WAEC Subjects
Aptitude Tests