Which of the following gasses is highly soluble in water at room temperature
ammonia
carbon (IV) oxide
chlorine
nitrogen
Correct answer is A
No explanation has been provided for this answer.
The formation of ethene from dehydration of ethanol can be described as-------
an addition reaction
an elimination reaction
an oxidation reaction
a substitution reaction
Correct answer is B
No explanation has been provided for this answer.
NaCl and KNO\(_{3}\)
KCL and NaNO\(_{3}\)
K\(_{2}\)SO\(_{4}\) and BaCl\(_{2}\)
NH\(_{4}\)NO\(_{3}\) and CO\(_{3}\)
Correct answer is C
when potassium sulfate is mixed with barium chloride a white precipitate of barium sulfate will be form. barium sulfate is slightly soluble in water, so it will precipitate out as a solid
BaCl2 + K2SO4 → BaSO4 + 2KCl
note; all substances are aqueous except BaSO4 which is solid
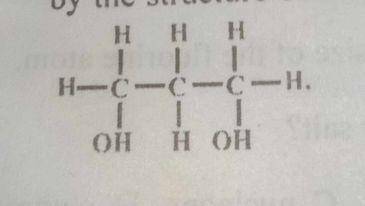
The alkanol represented by the structure above is
primary and dihydric
secondary and monohydric
tertiary and dihydric
secondary and dihydric
Correct answer is A
It is primary alcohol because it has only one alkyl group and dihydric because it has two hydroxyl groups
Give the common name for the following compound (CH\(_{3}\))\(_{2}\) CH CH\(_{2}\) -Br
Isobutyl bromide
Methyl bromide
propyl bromide
Butyl bromide
Correct answer is A
Common name : Isobutyl bromide
IUPAC name : 1-Bromo-2-methylpropane
Boiling point : 102°C
Formula : C\(_4\)H\(_9\)Br
WAEC Subjects
Aptitude Tests