The figure above shows the electrolysis of molten sodium ...
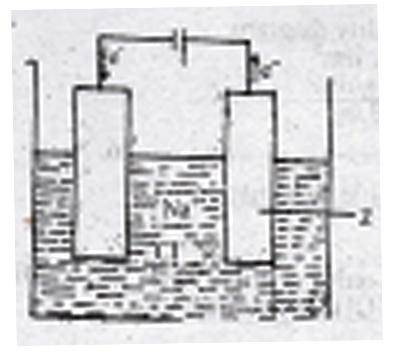
The figure above shows the electrolysis of molten sodium chloride. Z is the
anode where the Cl\(^-\) ions are oxidized
cathode where the Cl\(^-\) ions are reduced
anode where the Na\(^+\) ions are reduced
cathode where the Na\(^+\) ions are oxidized
Correct answer is A
During the electrolysis of brine, the chlorine ions are discharged at the anode and are oxidized. While at the cathode Hydrogen ion is discharged.
Similar Questions
Which of the following molecules has a linear shape? ...
A particle that contains 11 protons, 12 neutrons and 10 electrons is probably a ...
A major effect of oil pollution in coastal waters is the? ...
Which of the following will change when a catalyst is added to a chemical reaction? ...
Which of the following groups contains entirely linear molecules? ...